Chapter 5a
Chemical Principles
Basics of Chemistry
element electron
atom ion
proton cation
/ anion
neutron molecule
Parts of an Atom
Atom the smallest
unit of matter that displays all of the properties of the element
Nucleus - the center of the atom, with
most of the mass
composed
of:
protons
positively charged particles (+)
neutrons neutrally charged particles
(0)
correct pronunciation: “nuclear” NOT “nucular”
Electrons - negatively (-) charged
particles that orbit the nucleus in discrete shells
An electrically stable atom has the same number of
protons as electrons
the
charges balance (+) = (-)
nuclei are small: 10–15
meters, 1.67 x 10–27
kilograms
electrons are smaller: 10–19 meters, 9.1 x 10–31 kilograms
Elements
An element is defined by the number of protons
in the nucleus
Examples:
1 proton hydrogen
6 protons carbon
8 protons oxygen
26 protons iron
92 protons uranium
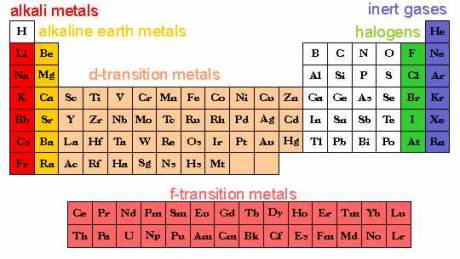
The Periodic Table of the Elements
A way of organizing the elements to show
their physical properties
elements identified by atomic
number
Electron Shells
Determine chemical
reactions
Electrons orbit the nucleus
in specific shells
Each shell has a maximum number
of electrons, which is its stable configuration
An atom is unstable if it has only one or two
electrons in the outer shell,
or if it
is missing one or two electrons in the outer shell

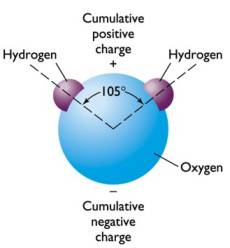
For example, hydrogen and oxygen combine to form water (H2O):
The first two shells are
filled by 2 and 8 electrons, respectively.
Hydrogen has one electron in the inner shell, and needs to add a second electron to be stable
Oxygen has 2 electrons in the inner shell (which is stable), but only 6 in the second shell, and needs to add 2 electrons to be stable
If oxygen “shares” electrons with 2 hydrogen atoms, the outer shell of the hydrogen has 2 electrons and is stable, and the outer shell of oxygen has 8 electrons and is stable
This sharing of electrons forms a covalent bond between the oxygen and hydrogen
atoms
Ions
if the charges on an atom
don’t balance:
cation – a positive ion, “missing” one or
more electrons
{ cat ion }
most metals produce cations
example: Na+
anion – a negative ion, with one or more
“extra” electrons
{ an ion }
example: Cl–
Elemental cations and elemental anions
the
configuration of the electron shells determines the preferred ionic charge for
an element
Elemental cations
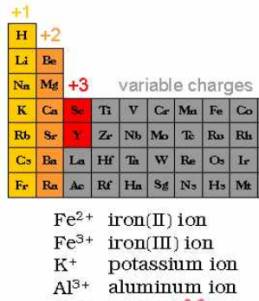
Elemental
anions
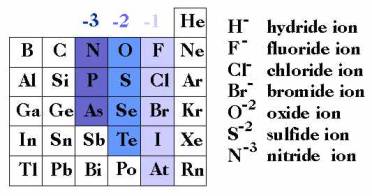
Groups of elements with similar properties
also determined by the
electron shells